Ion exchange resin is a polymer material with ion exchange function, which is widely used in water treatment, chemical processing and power generation. In water treatment, it can effectively remove various ionic impurities in water and produce high-quality water that meets different needs; in chemical processing and power generation, it also undertakes key ion separation and purification tasks.
However, as the resin continues to adsorb ions during use, its exchange capacity will gradually be exhausted. At this time, regeneration becomes an important part of maintaining the effectiveness of the resin and the efficiency of system operation. Reasonable regeneration operation can not only ensure the continuous and stable operation of the system, but also reduce production costs.
This guide is suitable for plant operators, facility engineers and water treatment professionals, aiming to provide a systematic and practical knowledge system for ion exchange resin regeneration.
1.What is Ion Exchange Resin Regeneration?
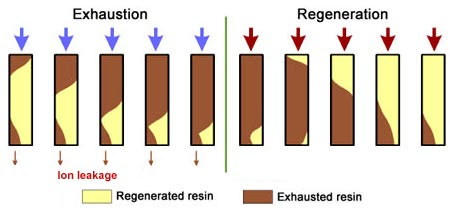
Ion exchange resin regeneration refers to the process of restoring the exchange capacity of the resin through specific chemical treatment methods after the ion exchange capacity of the resin is exhausted. Through regeneration, the resin can regain the ability to remove ions from water, which is crucial to maintaining stable water quality and avoiding system downtime caused by resin failure.
When there are signs of ion exchange resin exhaustion, regeneration should be considered. Common signs include increased total dissolved solids (TDS) in the water, reduced water flow rate, and decreased ion removal efficiency.
In actual operation, a variety of monitoring tools can be used to determine whether the resin needs to be regenerated. For example, using a conductivity sensor to monitor changes in water conductivity, using a hardness test kit to detect the hardness ion content in the water, and using a pressure gauge to monitor changes in water flow pressure can help operators detect changes in resin performance in a timely manner and determine the best time to regenerate.
2.Main Types Of Ion Exchange Resins And Their Regeneration Methods
Cation Exchange Resins
Cation exchange resins are mainly used to remove positively charged ions in water, such as calcium ions (Ca²⁺), magnesium ions (Mg²⁺), ferrous ions (Fe²⁺), etc. In the water treatment softening process, it can effectively reduce the hardness of water.
Its regeneration usually uses sodium chloride (NaCl), hydrochloric acid (HCl) or sulfuric acid (H₂SO₄). Among them, sodium chloride is often used for the regeneration of ordinary softening resins; hydrochloric acid and sulfuric acid are suitable for the regeneration of strong acid cation exchange resins, which can more effectively replace the cations adsorbed on the resin.
Anion Exchange Resins
Anion exchange resins are responsible for removing negatively charged ions in water, such as chloride ions (Cl⁻), sulfate ions (SO₄²⁻), nitrate ions (NO₃⁻), etc. In deep water treatment, anion exchange resins can further purify water quality.
Its regeneration generally uses sodium hydroxide (NaOH) or ammonia solution. Sodium hydroxide is a commonly used strong alkaline regeneration agent that can undergo a replacement reaction with anions adsorbed on the resin to restore the exchange capacity of the resin.
strong>Mixed Bed Resins>
Mixed bed resins contain both cationic and anionic resins and are usually used in polishing processes that require extremely high water quality. Due to its internal structure and working characteristics, the regeneration of mixed bed resins is relatively complicated and may require a separate regeneration process or the resins to be sent to a professional organization for regeneration.
3.Regeneration Process Step By Step
Backwashing
Backwashing is the first step of the regeneration process. Its main function is to remove particulate matter in the resin bed and redistribute the resin particles evenly. During the backwashing process, water flows upward from the bottom of the resin bed to remove impurities deposited on the surface and inside of the resin.
Generally speaking, the duration of backwashing is 15-30 minutes, and the specific time needs to be adjusted according to the type of resin and the degree of contamination. The flow rate of backwashing is usually controlled to expand the resin layer by 30%-50% to ensure effective removal of impurities without damaging the resin.
Chemical Agent Injection
Depending on the type of resin, the corresponding regenerant solution is injected. During the injection process, the concentration, volume and contact time of the regenerant need to be considered.
The appropriate regenerant concentration can ensure that the regeneration reaction is fully carried out. Too high or too low concentration may affect the regeneration effect; the regenerant volume needs to be determined according to the resin loading and exchange capacity; sufficient contact time allows the regenerant to fully react with the resin, and the general contact time is 30-60 minutes.
>Displacement Flushing (Slow Flushing)
During the displacement flushing stage, flushing is carried out at the same flow rate as the chemical injection, with the purpose of pushing out the excess regenerant in the resin bed to ensure that the ion exchange reaction is complete. Slow flushing can further improve the regeneration effect and reduce the impact of residual regenerant on subsequent operation.
Fast Flushing
Fast flushing is the last step of regeneration, and flushing is carried out at the normal flow rate during system operation until the water quality reaches the stable standard. This step is to completely remove the residual chemical agents in the resin, restore the resin to a state that can be used normally, and ensure the quality of subsequent treated water.
Factors Affecting Regeneration Efficiency
Water temperature and water flow rate have a significant impact on regeneration efficiency. Appropriate water temperature (generally 20-30℃) can accelerate the ion exchange reaction rate and improve the regeneration effect; while too fast or too slow water flow rate may cause insufficient contact between the regenerant and the resin, reducing the regeneration efficiency.
The quality and accurate dosing of the regenerant are also crucial. Regenerants with high purity and few impurities can effectively avoid resin contamination, and accurate dosing can ensure that the regeneration reaction is fully carried out and reduce waste.
In addition, the age of the resin, whether it is contaminated by organic matter, iron or microorganisms, and the design and operation cycle of the ion exchange column will all have an impact on the regeneration efficiency. The exchange groups of resins that have been used for a long time may partially fail; and contaminated resins will be more difficult to regenerate; reasonable ion exchange column design and operation cycle arrangement will help improve the regeneration effect and resin service life.
4.Advantages And Disadvantages Of Common Regeneration Chemicals
|
Chemical Reagent |
Application |
Advantages |
Limitations |
|
Sodium Chloride (NaCl) |
Softening treatment |
Low cost, high safety |
Less effective for high-hardness water treatment |
|
Hydrochloric Acid (HCl) |
Regeneration of strong acid cation resin |
Significant regeneration effect |
Corrosive; requires strict safety protection measures |
|
Sodium Hydroxide (NaOH) |
Regeneration of strong base anion resin |
Strong regeneration capability |
Corrosive; high operational risk |
|
Sulfuric Acid (H₂SO₄) |
Cation resin regeneration |
Cost-effective |
Risk of calcium sulfate precipitation |
5.Recommendations for Optimizing Resin Life And Regeneration Cycle
Using high-purity regeneration agents can effectively prevent impurities from contaminating the resin and extend the service life of the resin. Regularly using cleaning agents such as citric acid to clean the resin can effectively remove problems such as iron contamination.
During the regeneration process, be careful to prevent air from entering the system. Air entry may cause resin oxidation, dry columns in the resin layer, and other problems, affecting resin performance and regeneration effects.
In addition, regular performance diagnosis and testing of the resin is also necessary. By testing the exchange capacity, mechanical strength and other indicators of the resin, the state of the resin can be understood in a timely manner, providing a basis for optimizing regeneration operations and determining the timing of resin replacement.
6.When Should Ion Exchange Resins Be Replaced Rather Than Regenerated?
When the resin is physically damaged, such as resin particle cracks, significant color changes, and resin expansion and deformation, it means that the resin structure has been destroyed and it is difficult to restore performance through regeneration. At this time, the resin should be replaced.
If the resin is irreversibly contaminated, such as oil, heavy metals, organic matter, etc., and the contaminants cannot be removed by conventional cleaning and regeneration methods, it also needs to be replaced.
Even after a complete regeneration process, the performance of the resin still cannot meet the use requirements, and the frequent occurrence of problems such as substandard water quality also means that the resin has lost its use value and should be replaced in time.
7.Conclusion
Ion exchange resin regeneration is a key link to ensure the efficient and stable operation of the system. Through reasonable regeneration operations and strict adherence to the regeneration steps, the ion exchange capacity of the resin can be effectively restored, the service life of the resin can be extended, the operating cost can be reduced, and the water quality can be ensured to be stable and meet the standards.
For plant operators, facility engineers and water treatment professionals, mastering the knowledge and operating skills of ion exchange resin regeneration is of great significance to improving the overall performance and economic benefits of the system.


.png)

