For operators and technical personnel in the electroplating industry, efficiently treating wastewater containing heavy metals and complying with increasingly stringent environmental standards is crucial for ensuring long-term compliant operations.
Among various treatment technologies, ion exchange technology has become the preferred solution for advanced treatment of electroplating wastewater due to its excellent removal efficiency and resource recovery potential. This article will delve into which ion exchange resins are best suited for removing heavy metals from electroplating wastewater, combining technical principles, resin characteristics, and application logic to provide professional reference for industry professionals.
Expert Insight from COMCESS
As a professional supplier specializing in ion exchange and chelating resins for industrial wastewater treatment, COMCESS has long supported electroplating plants worldwide in achieving ultra-low heavy metal discharge and metal recovery.
Based on extensive project experience in copper, nickel, zinc, and mixed-metal electroplating wastewater, the following analysis combines practical engineering experience with resin selection logic, rather than theoretical discussion alone.
.png)
1.Why Heavy Metal Removal is Crucial in Electroplating Wastewater
Electroplating wastewater is complex in composition and contains a large number of heavy metal ions. These heavy metals are highly toxic to the environment and cannot be biodegraded. Once they enter water bodies and soil environments, they accumulate through the food chain, causing irreversible harm to ecosystems and human health, and also posing a direct threat to the compliant operation of enterprises.
Main Characteristics of Electroplating Wastewater
The electroplating process requires the use of various plating solutions, additives, brighteners, and other chemical agents, resulting in the wastewater exhibiting significant complexity. On the one hand, the types of heavy metals are diverse and their concentrations fluctuate widely, ranging from tens of milligrams per liter to micrograms per liter; on the other hand, the wastewater pH is often in extreme ranges (strongly acidic or strongly alkaline), and is accompanied by a large number of organic complexing agents, surfactants, and other components, further exacerbating the treatment difficulty.
Common Heavy Metal Pollution
In a typical electroplating workshop, the wastewater usually contains heavy metal ions such as copper (Cu), nickel (Ni), zinc (Zn), lead (Pb), cadmium (Cd), and cobalt (Co). Among them, copper and nickel are widely present in the rinsing water of copper plating and nickel plating processes, while lead and cadmium mostly come from special electroplating processes or raw material impurities. These ions exist in both free and stable complexed forms with EDTA, cyanide, etc., posing different challenges to treatment.
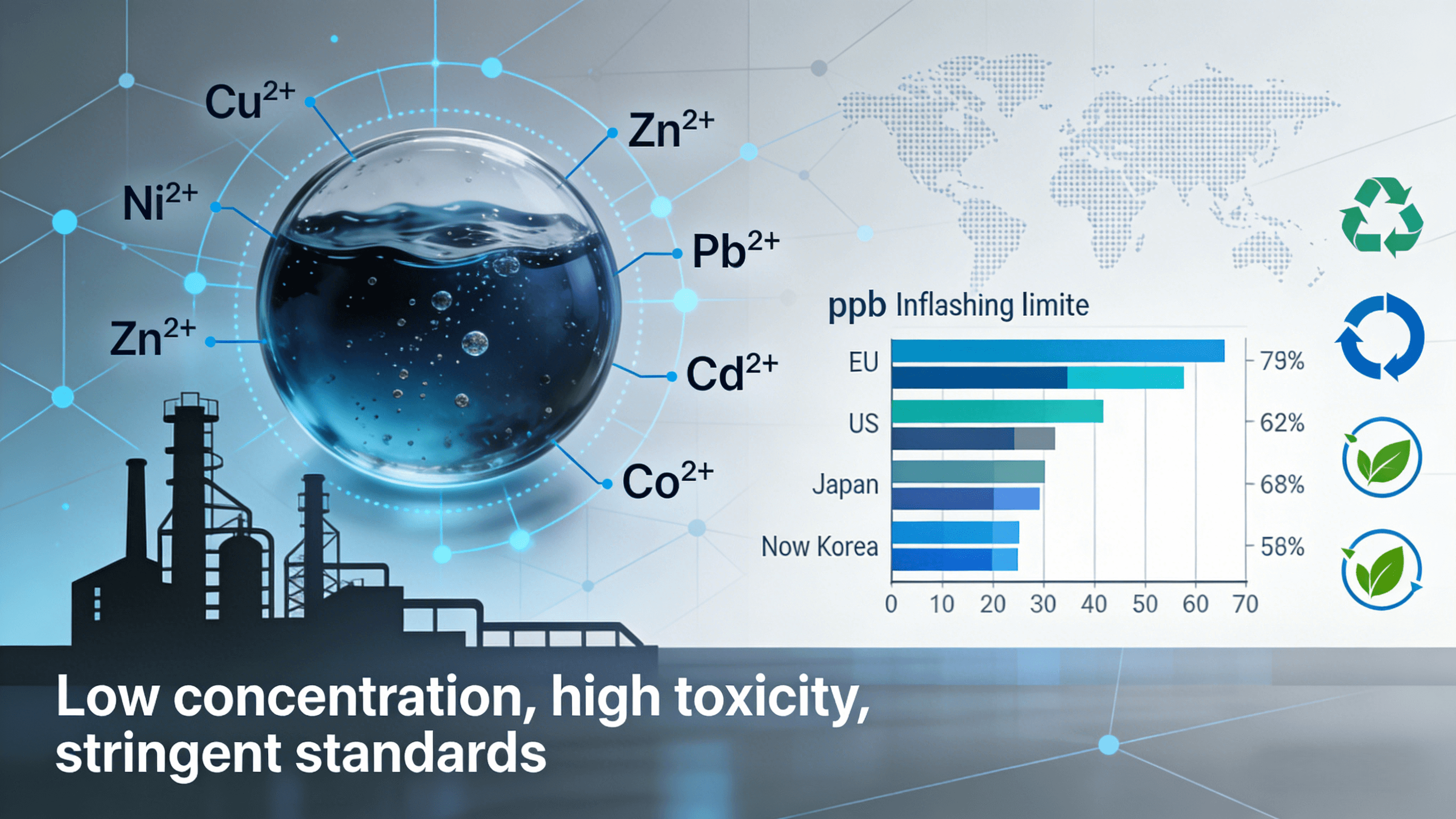
Environmental Regulations and Emission Limits
Global environmental regulations are continuously being upgraded, and the limits on heavy metal emissions from electroplating wastewater are becoming increasingly strict in various countries. Currently, regions such as Europe, the United States, Japan, and South Korea have tightened the emission limits for heavy metals such as copper and nickel to the ppb (micrograms per liter) level, and some regions even require near-zero emissions. Traditional treatment methods struggle to consistently meet this standard, and companies must adopt more efficient advanced treatment technologies to meet compliance requirements.
Limitations of Traditional Chemical Precipitation Methods
Chemical precipitation, as a traditional heavy metal treatment process, has the advantages of low initial investment and simple operation, but it also has many inherent drawbacks. This method uses alkaline solutions and sulfides to precipitate heavy metals, but it cannot effectively treat complexed metals—complexing agents will firmly bind to heavy metal ions, hindering the precipitation reaction. In addition, the precipitation process generates a large amount of chemical sludge containing heavy metals, which is classified as hazardous waste. Subsequent disposal costs are high, and it does not allow for the recovery and reuse of heavy metals, which is inconsistent with the concept of a circular economy.
2. Understanding Heavy Metals in Electroplating Wastewater
To achieve efficient treatment of heavy metals in electroplating wastewater, it is necessary to first clarify the source and form of heavy metals and accurately grasp the core challenges in the treatment process, so as to select appropriate treatment technologies and resin types.
Sources of Heavy Metals in Electroplating Processes
Cleaning Water Pollution
This is the main source of electroplating wastewater, accounting for more than 80% of the total wastewater volume. After electroplating, the workpieces need to undergo multi-stage rinsing to remove the electroplating solution attached to the surface. Each rinsing stage carries away a large amount of heavy metal ions. Although the heavy metal concentration in the rinsing water is lower than that of the original electroplating solution, the cumulative pollution load is extremely high due to the large discharge volume, making it a key target for treatment.
Tank Solution Carryover and Leakage
When workpieces are removed from the electroplating tank, a certain amount of electroplating solution will be adsorbed on the surface. If not adequately pre-treated for recovery, it will be directly carried into the rinsing system; at the same time, factors such as poor sealing of the electroplating tank, aging pipelines, and improper operation may also lead to leakage of the electroplating solution, causing a sudden surge in heavy metal concentration in the wastewater and impacting the treatment system.
Equipment Maintenance
The daily maintenance of electroplating equipment also generates wastewater containing heavy metals. For example, operations such as filter cartridge cleaning, anode bag replacement, plating tank cleaning, and pipeline flushing will introduce plating solution and precipitates attached to the equipment into the wastewater system. The heavy metal content and composition of this type of wastewater fluctuate significantly with the maintenance process, posing higher demands on the adaptability of the treatment system.
Challenges in Treating Electroplating Wastewater
Low Concentration, High Toxicity
The heavy metal concentration in electroplating rinse water is often in the milligram or even microgram range, but these low concentrations of heavy metals still possess strong bioaccumulation and toxicity. For example, a nickel ion concentration of only 0.1 mg/L can cause toxicity to aquatic organisms; long-term exposure to low concentrations of cadmium ions can cause serious damage to the human kidneys and skeletal system. This requires treatment technologies with high-precision removal capabilities.
Interference from Competing Ions
Electroplating wastewater usually contains a large number of harmless cations such as sodium (Na⁺), calcium (Ca²⁺), and magnesium (Mg²⁺), whose concentrations are often tens or even hundreds of times higher than those of heavy metal ions. During the treatment process, competing ions will compete with heavy metal ions for the exchange sites on the resin, occupying the resin's exchange capacity, leading to a decrease in heavy metal removal efficiency and an increase in resin regeneration frequency, thus increasing operating costs.
Complex Chemical Environment
The chemical environment of electroplating wastewater is extremely complex. The pH value often fluctuates below 2 or above 12, and changes in pH directly affect the existence form of heavy metal ions and the adsorption performance of the resin. At the same time, organic additives in the wastewater (such as complexing agents and brighteners) will form stable complexes with heavy metal ions, making it more difficult to adsorb and remove heavy metals; some process wastewater also contains components such as cyanide and ammonia nitrogen, further interfering with the stability of the treatment process.
3. Application of Ion Exchange Technology in Electroplating Wastewater Treatment
Ion exchange technology, with its precise ion selectivity, efficient removal capabilities, and resource recovery potential, has been widely used in the advanced treatment of electroplating wastewater. Its core advantage lies in its ability to specifically address the challenges of heavy metal removal under low concentration and complex matrix conditions.
Principle of Heavy Metal Removal by Ion Exchange
The essence of the ion exchange process is to utilize the functional groups on the surface of ion exchange resins to undergo equivalent exchange reactions with heavy metal ions in wastewater, adsorbing and fixing the heavy metal ions onto the resin while simultaneously releasing the original exchangeable ions in the resin (such as hydrogen ions or sodium ions), thereby achieving wastewater purification.
Basic Mechanism
The mechanism of ion exchange is mainly divided into two categories: firstly, electrostatic attraction, where the resin functional groups carry fixed charges and adsorb heavy metal ions with opposite charges through electrostatic interaction. This mechanism is common in ordinary ion exchange resins; secondly, chemical bonding, where the resin functional groups form stable chemical bonds (such as coordinate bonds) with heavy metal ions, resulting in stronger binding force and higher selectivity. This is the core mechanism of chelating resins.
Differences between Simple Ion Exchange and Chelation
Ordinary ion exchange (such as strong acid and weak acid cation exchange) mainly relies on electrostatic attraction to achieve ion exchange, only distinguishing the charge properties of ions (cations, anions), and cannot effectively distinguish different types of cations or anions, resulting in weaker selectivity. Chelating exchange, on the other hand, forms a cyclic chelate structure between the resin functional groups and heavy metal ions. This "chelation effect" gives the resin a very strong affinity for specific heavy metal ions, allowing it to preferentially adsorb heavy metal ions even in the presence of a large number of competing ions, resulting in stronger binding and more thorough removal.
Role of Functional Groups
The functional groups on the resin surface are crucial in determining its adsorption performance. Different functional groups have different chemical structures and electron distributions, leading to varying selectivity and adsorption capacity for heavy metal ions. For example, iminodiacetic acid groups have excellent selectivity for nickel and copper ions, aminophosphonic acid groups have good adsorption effects on various divalent heavy metal ions, and thiol groups have specific adsorption capabilities for heavy metals such as mercury and lead. Therefore, the selection of functional groups needs to be precisely matched to the heavy metal components in the wastewater.
Why Is Ion Exchange Widely Used In The Electroplating Industry?
Deep Purification Capability
Ion exchange technology can reduce the concentration of heavy metal ions in wastewater to ppb levels, far exceeding the treatment accuracy of traditional chemical precipitation methods. It can reliably meet stringent emission standards worldwide, providing reliable protection for companies to avoid environmental compliance risks. The deep purification advantages of ion exchange are particularly prominent in scenarios such as wastewater reuse and end-of-pipe polishing treatment.
Compact and Efficient Equipment
Compared to the large sedimentation tanks and reaction tanks required by traditional chemical precipitation methods, the ion exchange system uses a column structure, occupying a small footprint, only 1/5 to 1/3 of that of traditional processes, which is very suitable for the limited space in electroplating workshops. At the same time, the system can achieve automated control, ensuring stable treatment results and reducing labor costs by precisely adjusting parameters such as flow rate and regeneration cycle.
Resource Recovery Potential
After the ion exchange resin is saturated with adsorbed ions, its adsorption capacity can be restored through regeneration treatment. The eluent produced during the regeneration process contains high concentrations of heavy metal ions, which, after further treatment (such as electrolysis and evaporative concentration), can be recovered and reused in the electroplating process, achieving resource recycling. This integrated "treatment-recovery" model not only reduces the generation of hazardous waste but also reduces companies' reliance on virgin heavy metal raw materials, improving economic efficiency.
4. Chelating Ion Exchange Resins
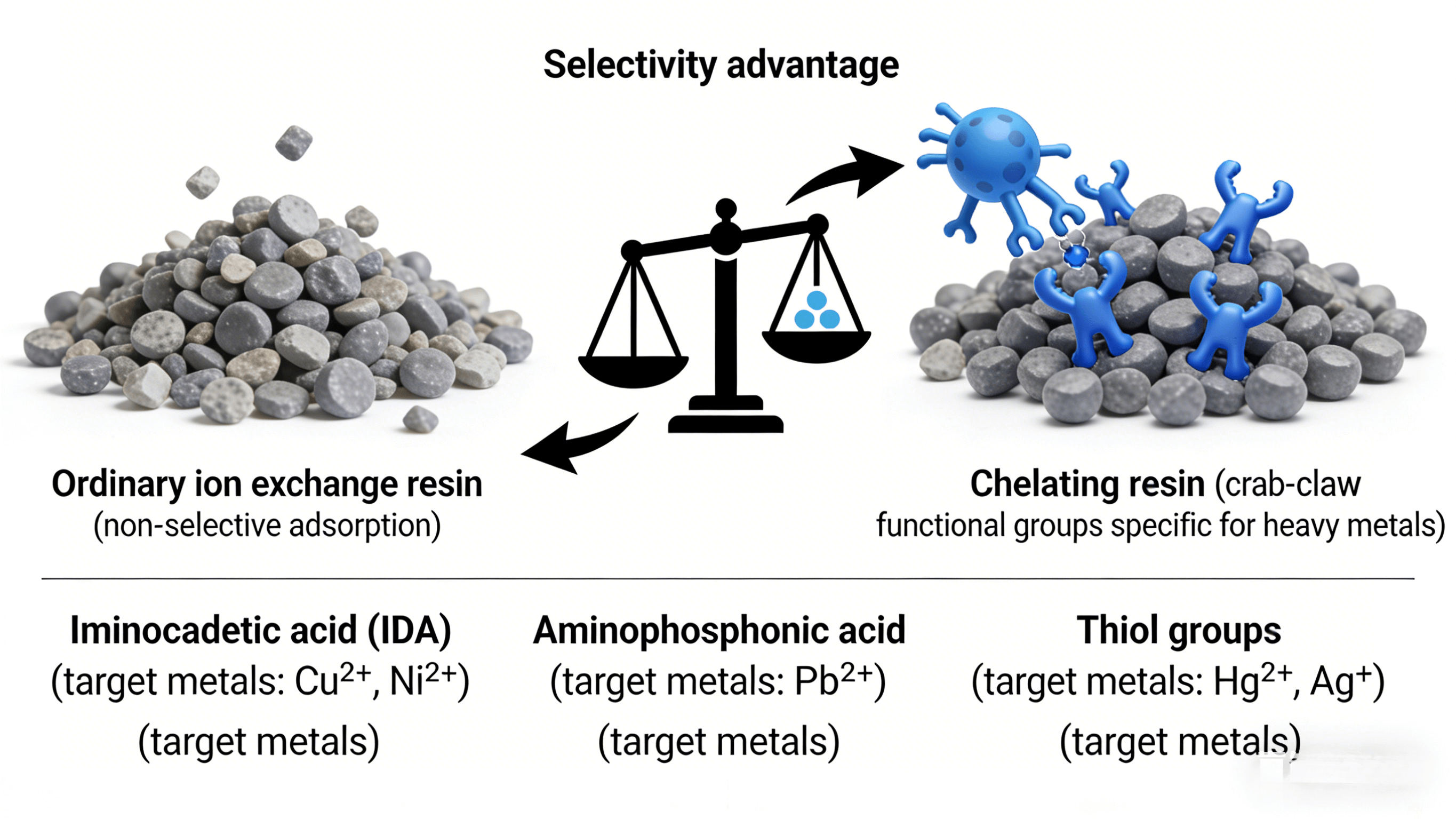
Given the complex water quality characteristics of electroplating wastewater, the need for low-concentration heavy metal removal, and the problem of competing ion interference, chelating ion exchange resins, with their high selectivity, strong stability, and excellent regeneration performance, have become the preferred type of resin for heavy metal treatment in electroplating wastewater.
What are Chelating Resins?
Chelating resins are a special type of ion exchange resin whose molecular structure contains functional groups that can form multi-coordinate complexes with heavy metal ions. Compared to ordinary ion exchange resins, they have stronger adsorption specificity and binding stability.
Structural Characteristics
The core structure of chelating resins consists of a polymer backbone and chelating functional groups. The polymer backbone (such as a polystyrene-divinylbenzene backbone) provides mechanical strength and chemical stability, ensuring that the resin can withstand complex operating conditions such as acids, bases, and high temperatures; the chelating functional groups act like "crab claws," containing two or more coordinating atoms in their molecular structure, which can form cyclic chelates with heavy metal ions, firmly "clamping" the heavy metal ions, making them difficult to be displaced by other ions.
Typical Functional Groups
There are three main types of chelating resin functional groups commonly used in electroplating wastewater treatment: firstly, iminodiacetic acid (IDA), which has extremely high selectivity for divalent heavy metal ions such as nickel, copper, and zinc, and strong acid resistance, making it the most widely used chelating functional group in the electroplating industry; secondly, aminophosphonic acid, which has a wider adsorption range and weaker adsorption capacity for calcium and magnesium ions, suitable for treating electroplating wastewater with complex compositions; and thirdly, thiol groups, which have specific adsorption capacity for heavy metal ions such as mercury, lead, and cadmium, and are often used in the treatment of mercury-containing and lead-containing electroplating wastewater.
Heavy Metals Effectively Removed by Chelating Resins
Copper (Cu²⁺)
Iminodiacetic acid and aminophosphonic acid type chelating resins have extremely strong affinity for copper ions. Even in acidic environments with a pH of 2-6, they can efficiently adsorb copper ions, with a removal rate of over 99.9%. They are particularly suitable for treating rinse water from copper plating processes, reducing the copper ion concentration to below 10 micrograms/liter, while the copper ion concentration in the regenerated eluent can reach tens of grams/liter, facilitating recycling.
Nickel (Ni²⁺)
Chelating resins are the core material of nickel recovery systems in electroplating. Among them, iminodiacetic acid type chelating resins have far superior selectivity for nickel ions compared to ordinary resins, and can preferentially adsorb nickel ions even in the presence of large amounts of sodium and calcium ions. In nickel plating rinse water recycling systems, chelating resins can achieve efficient nickel recovery, and the recovered nickel salts can be directly reused in the plating bath, significantly reducing raw material consumption.
Zinc, Lead, Cadmium, Cobalt
For heavy metal ions such as zinc (Zn²⁺), lead (Pb²⁺), cadmium (Cd²⁺), and cobalt (Co²⁺), chelating resins can achieve high-precision removal. Among them, aminophosphonic acid-type chelating resins have excellent adsorption effects on zinc ions, while thiol-based chelating resins have stronger selectivity for lead and cadmium ions. The appropriate resin can be selected based on the wastewater composition to ensure that the heavy metal concentration after treatment meets discharge or reuse requirements.
Tailored Resin Selection Matters
Different electroplating lines generate very different wastewater profiles. Selecting the wrong chelating resin can lead to premature saturation, high regeneration frequency, or unstable effluent quality.
Comcess provides application-oriented guidance to help customers match the right chelating resin type to specific metals, pH conditions, and complexing agents.
.png)
Key Advantages in Electroplating Wastewater Treatment
High Selectivity and Strong Anti-interference Ability
The greatest advantage of chelating resins is their high selectivity for heavy metal ions. In electroplating wastewater containing high concentrations of sodium, calcium, and magnesium salts, they can preferentially adsorb heavy metal ions, almost unaffected by competing ions. This characteristic prevents the resin's exchange capacity from being occupied by useless ions, extending the resin's operating cycle and reducing regeneration frequency and chemical consumption.
Wide Operating Condition Adaptability and Strong Stability
Most chelating resins maintain stable adsorption performance within a pH range of 2-11, adapting to the large pH fluctuations of electroplating wastewater without the need for additional pH adjustment, simplifying the treatment process. At the same time, chelating resins have high mechanical strength and good chemical stability, can withstand the erosion of acid and alkali regenerants, and have a service life of 3-5 years, far exceeding that of ordinary resins.
Precise Polishing, Suitable for Advanced Treatment
Chelating resins are very suitable as a "polishing" unit in electroplating wastewater treatment processes, used for advanced treatment after conventional treatment processes (such as chemical precipitation and ordinary filtration). Through chelating resin polishing, trace heavy metal ions remaining in the wastewater can be completely removed, ensuring that the effluent water quality meets reuse standards or the most stringent discharge standards, providing a guarantee for enterprise wastewater resource utilization.
Regeneration and Metal Recycling Potential
The regenerability of chelating resins is a core prerequisite for their economical and environmentally friendly operation. Through scientific regeneration processes, not only can the resin's adsorption capacity be restored, but heavy metals can also be recovered and reused.
Regeneration Principle
When the chelating resin is saturated with adsorbed metals, strong acids such as sulfuric acid and hydrochloric acid are typically used as regeneration agents. During the regeneration process, high concentrations of hydrogen ions (H⁺) react with the heavy metal ions on the chelating functional groups of the resin, breaking the chelate structure and desorbing the heavy metal ions into the regeneration solution. The resin is then restored to its hydrogen form, regaining its adsorption capacity and enabling cyclic reuse. The concentration, flow rate, and contact time of the regeneration agent need to be precisely adjusted according to the resin type and heavy metal species to ensure effective regeneration.
Environmental and Economic Benefits
The eluent produced during regeneration contains high concentrations of heavy metal ions, reaching tens of grams per liter. These can be recovered as high-purity elemental metals through electrolytic refining, or concentrated by evaporation to produce metal salts for reuse in electroplating processes, achieving resource recycling. This model not only reduces the generation and disposal costs of heavy metal waste but also lowers the enterprise's demand for virgin metal raw materials, balancing environmental and economic benefits and aligning with the concept of green production.
5. Other Resin Options and Their Limitations
In addition to chelating ion exchange resins, strong acid cation exchange resins (SAC) and weak acid cation exchange resins can also be used for heavy metal treatment. However, due to inherent performance limitations, they are only suitable for specific scenarios and cannot meet the advanced treatment demands of complex electroplating wastewater conditions.
Strong Acid Cation Exchange Resins (SAC)
Core Characteristics and Application Scenarios
Strong acid cation exchange resins feature sulfonic acid groups (-SO₃H) as functional units, exhibiting strong acidity and high ion exchange capacity. They operate stably in acidic, neutral, and alkaline environments. Their mechanism involves exchanging hydrogen ions with cations in wastewater, adsorbing all cations including heavy metals, sodium, calcium, and magnesium. They are commonly used for water softening and primary desalination.
Limitations
The primary drawback of this resin type is its lack of selectivity. It cannot distinguish between heavy metal ions and competing ions like sodium, calcium, and magnesium. In electroplating wastewater, abundant competing ions preferentially occupy the resin's exchange sites. This results in low heavy metal removal efficiency, frequent resin regeneration, and high operational costs. Additionally, its adsorption capacity for complexed heavy metal ions is extremely weak, rendering it ineffective for treating electroplating wastewater containing complexing agents. Consequently, it is only suitable for simple wastewater treatment with extremely high heavy metal concentrations and no competing ion interference, and is not applicable for advanced treatment of electroplating wastewater.
Weak Acid Cation Exchange Resin (WAC)
Core Characteristics and Application Scenarios
Weak acid cation exchange resins feature carboxyl (-COOH) functional groups, with adsorption performance highly dependent on pH. They effectively adsorb cations only in neutral or alkaline environments above pH 5. They exhibit stronger adsorption capacity for divalent cations (e.g., copper, nickel, calcium, magnesium ions) than monovalent cations (e.g., sodium ions), demonstrating selectivity. They are commonly used for softening high-alkalinity, high-hardness wastewater and primary removal of heavy metals.
Limitations
This resin type has a narrow application range and is significantly constrained by pH—in the acidic environments common in electroplating wastewater, carboxyl groups struggle to ionize, leading to a substantial decline or even loss of adsorption capacity. Additionally, they exhibit limited treatment capacity for complexed heavy metal ions and inferior resistance to competitive ion interference compared to chelating resins. Their treatment stability in complex electroplating wastewater is poor, limiting their role to primary treatment units that cannot meet stringent compliance requirements.
6. Key Factors for Selecting Appropriate Resins
Resin selection directly determines the treatment effectiveness, operational costs, and compliance of electroplating wastewater treatment. It requires comprehensive consideration of multiple dimensions, including wastewater composition, operating conditions, and system design, to avoid suboptimal treatment outcomes and cost wastage due to blind selection.
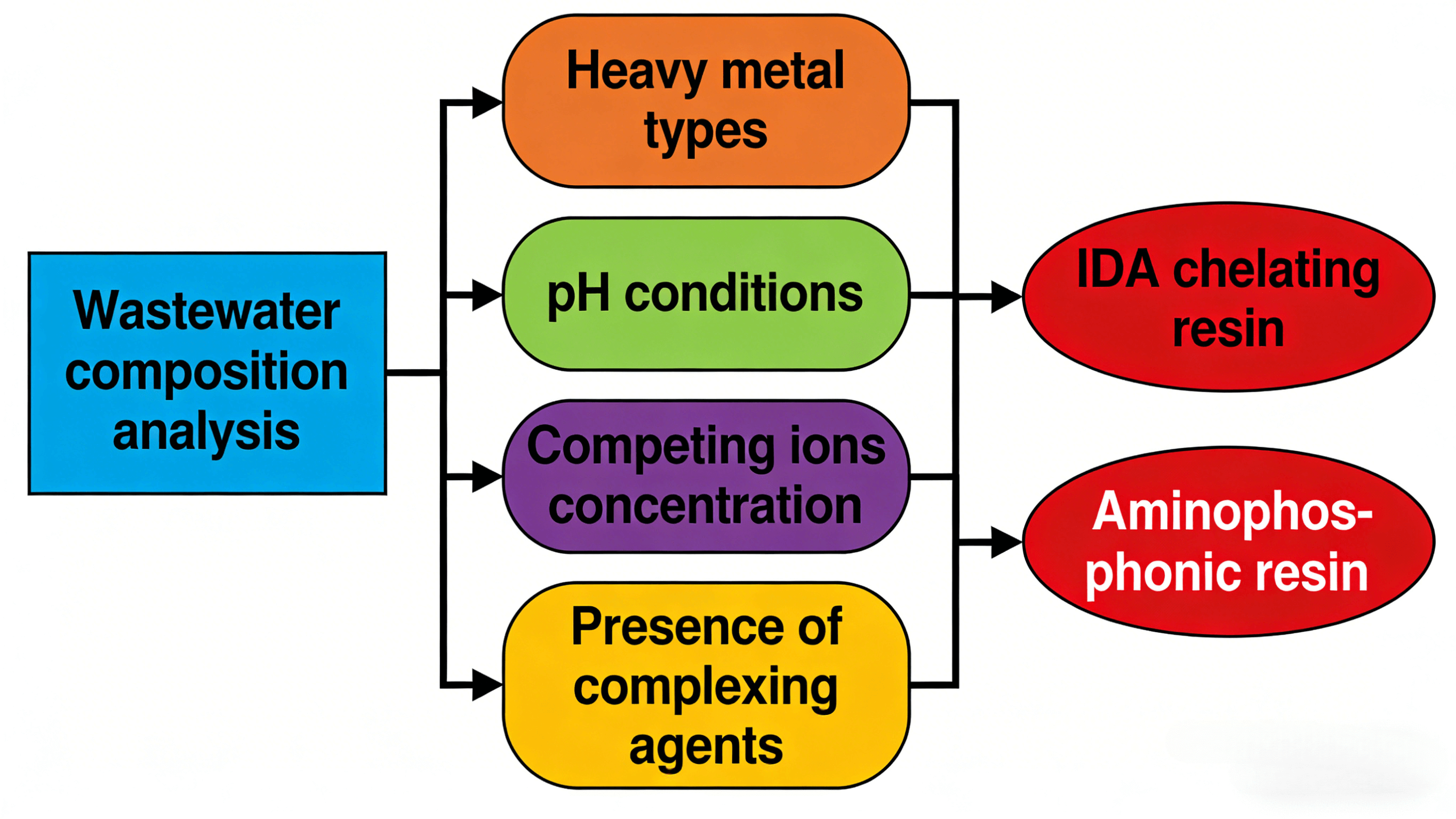
Comprehensive Wastewater Composition Analysis
Wastewater composition analysis is the core prerequisite for resin selection. Precise testing must clarify the following key indicators: First, the types, concentrations, and forms (free or complexed) of heavy metal ions. If strong complexing agents like EDTA or cyanide are present, chelating resins capable of adsorbing complexed heavy metals should be selected. Second, the concentration of coexisting impurities, including competing ions like sodium, calcium, and magnesium, as well as suspended solids, oils, and organic pollutants. These impurities affect resin adsorption performance and service life. Third, fundamental parameters such as wastewater pH, temperature, and turbidity, which provide the basis for resin selection and process design.
Operational Condition Compatibility Assessment
pH and Temperature
Optimal operating pH and temperature ranges vary across resin types and must be selected based on actual wastewater conditions. For example:
Iminodiacetic acid-type chelating resins operate best at pH 2–6 and temperatures below 60°C.
Strong acid cation exchange resins tolerate broader pH ranges but accelerate resin degradation at high temperatures. If wastewater pH or temperature fluctuates significantly, select more adaptable resins or install a conditioning unit prior to treatment.
Flow Rate and Contact Time
The empty tower velocity (BV/h) and contact time in the resin column directly impact adsorption efficiency. Excessively high flow rates result in insufficient contact between heavy metal ions and the resin, reducing removal efficiency. Conversely, excessively low flow rates decrease treatment capacity and increase equipment investment. Typically, the optimal empty tower velocity for chelating resins ranges from 10 to 30 BV/h. A reasonable contact time must be calculated based on the resin's adsorption kinetics and the heavy metal concentration in the wastewater to ensure complete adsorption of heavy metal ions.
Scientific Considerations in System Design
Equipment Configuration Selection
In electroplating wastewater treatment, ion exchange systems predominantly adopt fixed-bed configurations, with dual-tower or triple-tower series designs being widely applied. This configuration enables a continuous operation mode of “one tower in operation, one tower undergoing regeneration, and one tower on standby.” This avoids system downtime during single-tower regeneration while enhancing treatment precision and operational redundancy, ensuring stable effluent quality. For high-volume scenarios, a multi-column parallel design can be adopted to improve treatment efficiency.
Pretreatment and Resin Protection
Before entering the resin column, wastewater must undergo thorough pretreatment: - Precision filtration (≥5 micron) removes suspended solids and particulates to prevent resin pore blockage. - Oil separation and demulsification processes eliminate grease to prevent resin surface contamination (“poisoning”). - For wastewater containing strong oxidants or high organic concentrations, additional reduction and degradation units are required to protect resin functional groups from degradation. Comprehensive pretreatment significantly extends resin lifespan and reduces operational costs.
Regeneration and Lifespan Management
Develop scientifically sound regeneration protocols based on resin type and wastewater pollution load, including parameters such as regenerant type, concentration, dosage, and flow rate. This prevents incomplete or excessive regeneration that degrades resin performance. Simultaneously, regularly monitor indicators such as resin exchange capacity and adsorption efficiency to predict resin lifespan. Timely replacement of aged resin ensures long-term stable system operation.
7. Typical Application Scenarios in the Electroplating Industry
Ion exchange technology (particularly chelating resin processes) finds extensive application in the electroplating industry, covering core processes such as rinse water reuse, end-of-pipe advanced treatment, and heavy metal recovery. This supports enterprises in achieving compliant, efficient, and green production.
Rinse Water Recycling
Rinse water constitutes a significant proportion of electroplating wastewater. After treatment with chelating resins, heavy metal ions in rinse water can be reduced to trace levels, meeting reuse standards for recycling into upstream rinsing processes. This reuse model significantly reduces fresh water consumption (achieving water savings of 60%-80%), lowers wastewater discharge volumes, and recovers heavy metals from rinse water. It integrates “water conservation-recovery-emission reduction,” serving as a core technological pathway for water conservation and emission reduction in the electroplating industry.
End-of-Pipe Wastewater Advanced Treatment
Serving as the “final defense” in end-of-pipe treatment for electroplating wastewater, chelating resin processes provide advanced polishing after conventional treatments like chemical precipitation and filtration. By precisely adsorbing residual trace heavy metal ions, they ensure stable ppb-level heavy metal concentrations in effluent, meeting the most stringent discharge regulations and mitigating environmental compliance risks for enterprises. This technology is particularly suitable for regions or industries with extremely high discharge requirements, safeguarding long-term operational compliance.
Heavy Metal Recovery and Resource Utilization
For high-value heavy metals like nickel and copper, the chelation resin adsorption-regeneration process concentrates and recovers metals from wastewater. These recovered metals can be converted into metallic elements or metal salts for reuse in electroplating production. For instance, after treating nickel plating rinse water with chelating resins, the nickel concentration in the regenerated eluate reaches 50-100 g/L. Electrolytic refining then recovers high-purity nickel plates with an efficiency exceeding 95%. This resource recovery model not only reduces raw material costs but also minimizes heavy metal waste generation, aligning with circular economy principles.
8. Chelating Resin vs. Traditional Treatment Methods
Compared to conventional chemical precipitation methods, the chelating resin process offers significant advantages in treatment precision, environmental performance, and economic efficiency. Specific comparisons are as follows:
|
Dimension of Comparison |
Chelating Ion Exchange Resin |
Chemical Precipitation Method |
|
Effluent Precision |
Extremely high (ppb level), can stably meet standards |
General (ppm level), difficult to treat trace heavy metals |
|
Sludge Production |
None or minimal, only generates small amounts of regeneration waste liquid |
Large amounts of chemical sludge, classified as hazardous waste, high disposal costs |
|
Automation Level |
High, easy to integrate with automated control systems, convenient operation |
Relatively low, requires manual adjustment of chemical dosage and pH, poor stability |
|
Footprint |
Small, vertical column installation saves floor space |
Large, requires sedimentation tanks, reaction tanks, and sludge storage areas |
|
Operating Costs |
Mainly regeneration chemical costs, resin is reusable, long-term costs are controllable |
Chemical costs + sludge disposal costs, high long-term operating costs |
|
Resource Recovery |
Enables heavy metal recovery and reuse, improving economic benefits |
Cannot recover heavy metals, serious resource wastage |
|
Complex Water Adaptability |
Strong, can treat complexed heavy metals, high resistance to interference |
Weak, cannot treat complexed heavy metals, greatly affected by water quality fluctuations |
9. Conclusion
Chelating ion exchange resins represent the optimal path for achieving deep compliance with environmental standards and resource recovery in electroplating wastewater treatment. Their core value lies in: ensuring absolute environmental compliance through high selectivity, and creating economic value through heavy metal recovery. By simply selecting the right resin and implementing scientific operation and maintenance, companies can transform environmental investments into a green competitive advantage that reduces costs and increases efficiency.
Work with a Reliable Chelating Resin Partner
With increasing regulatory pressure and the growing importance of resource recovery, choosing the right ion exchange resin partner is critical.
COMCESS supports electroplating enterprises worldwide with high-performance chelating resins, technical consultation, and long-term application support for heavy metal removal and recovery.
For resin selection guidance or project discussion, feel free to contact our technical team.
.png)




