1. Why Must Resins Be Pretreated?
Ion exchange resins play an indispensable role in many key fields such as water treatment, ultrapure water preparation, and industrial desalination. With their highly efficient ion exchange capacity, they achieve precise removal of impurity ions from water, providing compliant water quality for production and daily life.
Although ion exchange resins undergo preliminary treatment at the factory, this level of treatment is far from sufficient for formal operation. Systematic pretreatment of the resins before formal use is crucial.
Scientific pretreatment not only significantly improves the resin's performance, ensuring it fully realizes its exchange potential, but also effectively reduces system operational risks, avoids equipment failures and water quality fluctuations, and extends the resin's lifespan, reducing maintenance costs for enterprises.
2. What Risks Arise From Untreated Ion Exchange Resins?
(1) Initial Water Quality Failure
Untreated resin will release a large amount of residual impurities during initial operation, directly leading to abnormal effluent quality. Total organic carbon (TOC) will increase significantly, affecting the chemical stability of the water; conductivity will also fluctuate drastically, failing to maintain a stable acceptable range; simultaneously, residual ions in the resin will be continuously released into the water, causing excessive ion content in the effluent.
(2) System Equipment Contamination
Fine powder, debris, and organic impurities carried by the resin will adhere to the inner walls of pipes under the action of water flow, causing pipe contamination and blockage, affecting water flow efficiency. These impurities can also enter downstream precision equipment, causing internal blockage and affecting normal operation. Furthermore, a large amount of impurities will increase the workload of the front-end filter, causing it to clog frequently, reducing filtration efficiency, and increasing equipment maintenance frequency.
(3) Degraded Resin Performance
Improperly dry-packaged or stored resin may contain air bubbles or locally dehydrated areas. Direct use without pretreatment will prevent these areas from fully expanding during operation, making them prone to breakage under water flow impact and pressure changes. Meanwhile, impurities covering the resin exchange sites can prevent it from reaching the designed exchange capacity, resulting in a significant decrease in working efficiency and fluctuations in exchange capacity during operation, leading to overall instability in the operating state.
3. Main Objectives of Resin Pretreatment
- To thoroughly remove organic impurities (such as free monomers and oligomers) and inorganic impurities (such as chlorides and sulfates) remaining in the resin during production, eliminating their impact on water quality and resin performance.
- To remove fine powder, debris, and residual free monomers generated during resin transportation and handling, preventing these substances from contaminating system equipment.
- To fully wet resin particles, ensuring their internal pores open sufficiently for uniform expansion and preventing breakage during operation.
- To convert the resin to the corresponding ionic form (such as H-form, Na-form, OH-form, etc.) according to the specific system's operational requirements, ensuring it can directly perform ion exchange after being put into use.
- To reduce the risk of water quality contamination during the initial operation of the system from the source, ensuring that the effluent water quality quickly reaches qualified standards.
4. Common Methods of Resin Pretreatment (Classified by Industry Standards)
Backwash
Backwashing is a fundamental pretreatment step, its core purpose being to remove debris, fine powder, and surface-adhered mechanical impurities from the resin. During operation, the backwash intensity must be carefully controlled to maintain the resin bed expansion rate at 50%–70%. This expansion level allows impurities to fully detach from the resin surface while preventing excessive wear of resin particles. The backwash process must be continuous until the discharged backwash water is clear and transparent, with no obvious suspended solids or impurities.
Ion Conversion Treatment
Ion conversion treatment is a crucial step in adjusting the resin to the ionic form required by the system. Common conversion directions for cation resins are H-form and Na-form, while anion resins are mostly converted to OH-form and Cl-form.
Specific reagents are used during the conversion process. For example, hydrochloric acid (HCl) is commonly used for converting cation resins to H-form, sodium hydroxide (NaOH) can be used for converting anion resins to OH-form, and sodium chloride (NaCl) is mostly used for converting cation resins to Na-form or anion resins to Cl-form. Through conversion, it is ensured that the resin is immediately in optimal working condition after being put into operation, directly performing the target ion exchange function.
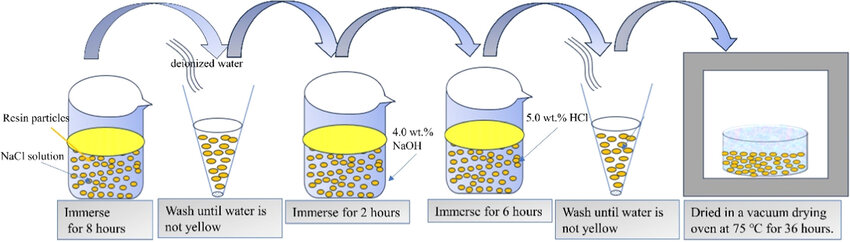
Acid/Alkali Treatment
Acid/alkali treatment is primarily used to remove stubborn impurities deeply attached to the resin bed, including organic contaminants and inorganic precipitates that are difficult to remove through backwashing. The typical process is "acid first, then alkali," meaning the resin is first soaked and circulated with a dilute acid solution, then the same operation is repeated with a dilute alkali solution. Multiple cycles can be performed if necessary to achieve the best cleaning effect.
This pretreatment method is widely used in desalination systems and ultrapure water preparation systems, effectively improving the exchange purity and stability of the resin.
Hot Water Rinse
The core function of hot water rinse is to reduce the content of soluble organic matter in the resin, thereby effectively controlling the TOC index of the effluent. During rinsing, the water temperature is typically controlled at 50–60°C. The resin bed is rinsed with hot water circulation, using the high temperature to promote the dissolution of soluble organic matter.
This method is particularly important in scenarios with extremely high water quality requirements, such as the pretreatment of electronic-grade and nuclear-grade resins, providing a more reliable resin guarantee for subsequent ultrapure water preparation.
Air Scour
Air scour is typically used as an adjunct to backwashing. By introducing compressed air into the resin bed, the resin particles experience more intense collisions and friction due to the combined action of air and water, thereby improving the efficiency of impurity removal.
This method is widely used in large-scale industrial ion exchange equipment and effectively enhances backwashing, especially suitable for treating resins containing a high concentration of impurities.
5. Pretreatment Differences in Different Application Scenarios
Boiler Softening Water Resin
The core requirement for boiler softening water systems is the removal of calcium and magnesium ions. Therefore, pretreatment focuses on backwashing for impurity removal and brine conversion. Typically, after backwashing to remove impurities, the cation exchange resin is converted to the Na form using sodium chloride solution; complex acid-base treatment is unnecessary, making the process relatively simple.
Industrial Desalination Resin
Industrial desalination systems require resins with high-efficiency ion exchange capacity to remove various anions and cations from water. Therefore, pretreatment must include acid-base washing steps to thoroughly remove deep-seated impurities. Simultaneously, the cation exchange resin must be converted to the H⁺ form, and the anion exchange resin to the OH⁻ form to ensure the resin can fully perform its desalination function.
Ultrapure Water Resin (Electronics and Photovoltaic Industries)
The electronics and photovoltaic industries have extremely stringent requirements for ultrapure water quality, with strict control over indicators such as TOC and ion content. Therefore, their resin pretreatment must undergo a complete acid-base treatment process, and it is recommended to add a hot water washing step to further reduce TOC content, ensuring that the resin can stably produce ultrapure water that meets standards after being put into use.
Nuclear Power or Specialty Resins
The nuclear power and specialty industries have extremely high requirements for the performance stability and safety of resins, and the pretreatment process is even more stringent. This typically requires a combination of processes such as multiple acid-alkali cycle cleaning and hot water cleaning. In some scenarios, special chemical treatments are also necessary to ensure that the resin is free of any potential impurities and performance risks.
6. Standard Flowchart for Resin Pretreatment (General Version)
- Resin Loading: Evenly pack new resin into the ion exchange column, avoiding resin particle accumulation or voids.
- Backwashing and Expansion: Purge with backwash water to expand the resin bed to the specified ratio, removing surface impurities and fine powder.
- Forward Wash to Clarity: Switch to forward washing and rinse the resin bed until the effluent is clear and transparent.
- Acid/Alkali or Salt Solution Injection: Inject the appropriate acid, alkali, or salt solution as needed for cleaning or conversion treatment.
- Slow Wash + Fast Wash: First, rinse the resin at a slower flow rate to ensure sufficient reagent reaction, then increase the flow rate for rapid rinsing to remove residual reagents.
- Water Quality Check: Test the conductivity, TOC, residual ions, and other indicators of the effluent to confirm compliance with requirements.
- Operation: After the water quality meets the standards, switch the resin system to normal operation.
7. Pretreatment Effect Evaluation Indicators
Evaluating the resin pretreatment effect requires attention to several core indicators. Among them, effluent conductivity is a fundamental indicator, directly reflecting the residual ions in the water; the lower the conductivity, the better the pretreatment effect. TOC reduction is crucial for measuring the removal of organic impurities, especially important for ultrapure water systems.
The content of residual ions (such as Na⁺ and Cl⁻) needs to be controlled at extremely low levels to avoid affecting subsequent water quality. Resin expansion uniformity reflects the resin's wetting and activation effect; uneven expansion may indicate potential performance issues. System pressure drop changes reflect the cleanliness and filling status of the resin bed; stable pressure drop indicates no significant blockage in the resin bed.
8. Conclusion
Ion exchange resin pretreatment is not optional, but a standard procedure that must be strictly followed.
It ensures that the resin enters the system in a clean, stable, and correct state, thereby guaranteeing high-quality effluent, reducing operational risks, and providing assurance for long-term operation.




